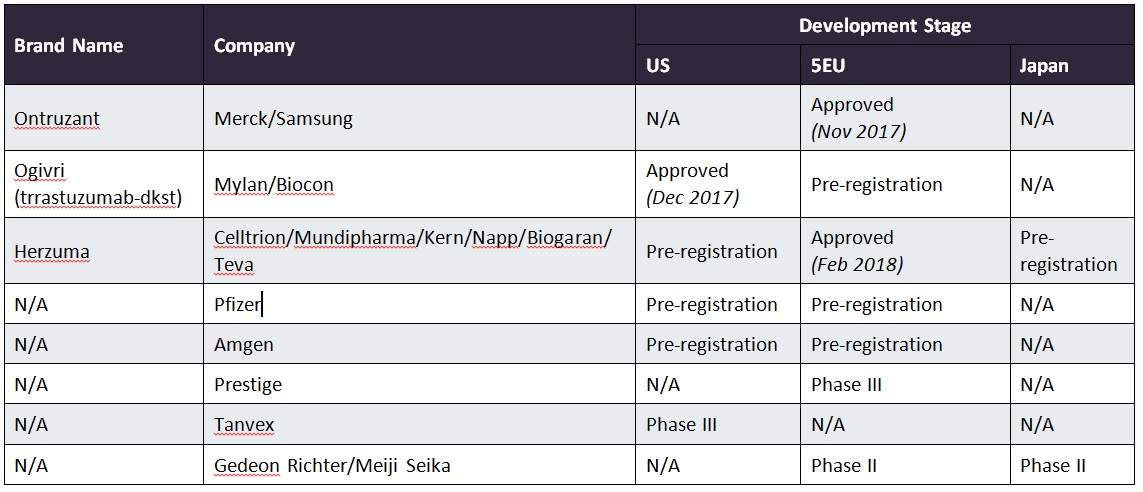
Celltrion and Teva Announce FDA Approval of Herzuma, a Biosimilar to Herceptin, for Treatment of HER2-Overexpressing Breast Cancer | Specialty Pharma Journal

Teva and Celltrion Healthcare Announce U.S. Availability of HERZUMA® (trastuzumab-pkrb) for Injection | Business Wire

Herzuma (trastuzumab biosimilar) for the Treatment of Breast and Gastric Cancers - Clinical Trials Arena

Celltrion and Teva's Herzuma (trastuzumab-pkrb- Herceptin biosimilar) Receives FDA's Approval for HER2-Overexpressing Breast Cancer
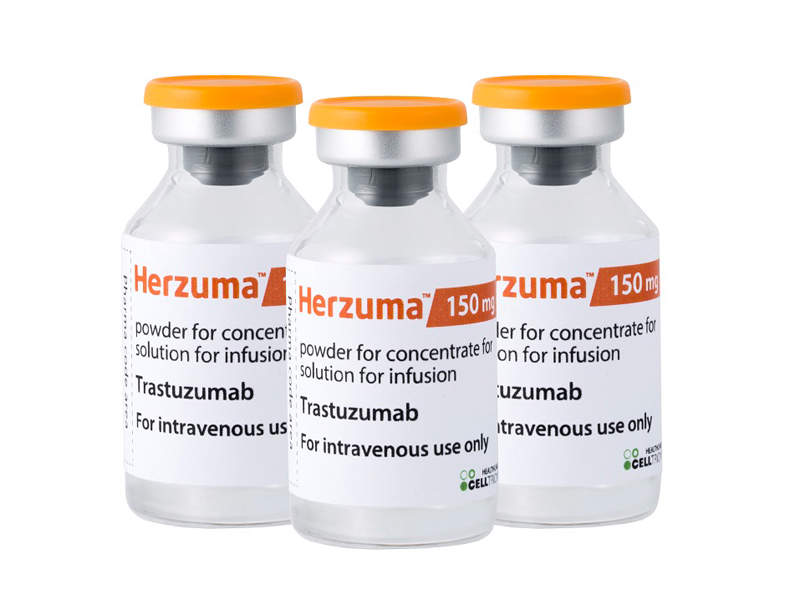
Herzuma (trastuzumab biosimilar) for the Treatment of Breast and Gastric Cancers - Clinical Trials Arena